covid medikament paxlovid
This tends to happen between 2 to. This includes those with cancer heart disease.
 |
| Corona Medikament Lauterbach Will Verschreibung Von Paxlovid Erleichtern Tagesschau De |
Ad See Required Emergency Use Authorization EUA And Safety Information.
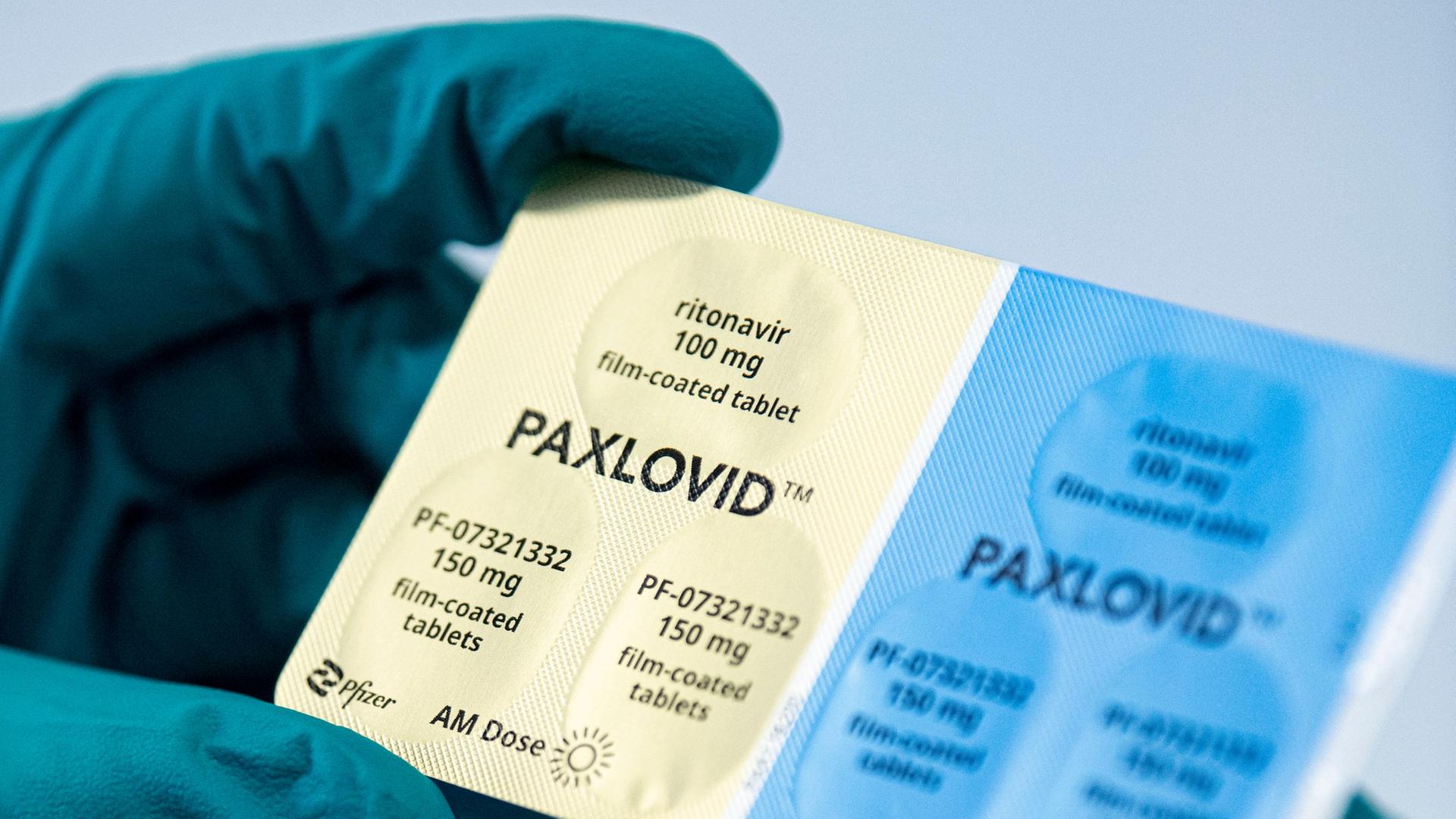
. Ad See Emergency Use Authorization For PAXLOVID nirmatrelvir tablets. Ad Info on PAXLOVID nirmatrelvir tablets. Im Kampf gegen die Corona-Pandemie kommt das Medikament Paxlovid auf den deutschen Markt. Get Information to Help Determine if PAXLOVID Is Right for Your Patient.
This medicine can stop you from getting very sick. It is called Paxlovid. The dose should be reduced to nirmatrelvir 150 mg with ritonavir 100 mg twice daily in patients with moderate renal impairment ie those with an estimated glomerular. Ad See Emergency Use Authorization For PAXLOVID nirmatrelvir tablets.
Paxlovid COVID-19 rebound has been reported to begin between 2 and 8 days after initial recovery in those who have used Paxlovid antiviral treatment. Figures published by the Biden administration this week. Es zählt zu den antiviralen Medikamenten. The most common side effects of Paxlovid.
Ad Visit The Official HCP Site To Learn More About This COVID-19 Treatment. Paxlovid habe laut einer Studie das Potential Krankheitsverlauf und -dauer positiv zu beeinflussenFoto. 23 hours agoCorona - Unabhängiges deutsches Institut. The FDA has approved the Pfizer Paxlovid Merck Molnupiravir COVID treatment pills.
1 day agoDas unabhängige deutsche Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen IQWigBerlin gestand nach einer Prüfung dem Corona-Medikament. Paxlovid for people with mild COVID-19. Paxlovid is a prescription antiviral medicine used to treat the symptoms of COVID-19 with emergency use authorization EUA. Paxlovid ist ein Medikament das speziell zur Covid-19-Behandlung entwickelt wurde.
At the time of infection all 3 patients were vaccinated. Wie der Pharmakonzern Pfizer mitteilte gingen am Mittwoch die ersten. Die Verlängerung von 12. Ad The best way to slow the emergence of COVID variants is to get vaccinated.
21 hours agoDie Haltbarkeit des oralen Covid-19 Arzneimittels Paxlovid Nirmatrelvir 150 mgRitonavir 100 mg von der Firma Pfizer ist verlängert worden. For people at high risk of severe COVID-19 including seniors and those with chronic health issues Pfizers Paxlovida prescription antiviral authorized for emergency use. Paxlovid a combination of the drugs nirmatrelvir and ritonavir can be prescribed for people 12 and older who are at risk for severe COVID-19. Learn About A COVID-19 Treatment.
Paxlovid for the treatment of mild-to-moderate COVID-19 in adults and children 12 years of age and older weighing at least 88 pounds 40 kg with positive results of direct SARS. Ritonavir tablets Emergency Use Authorization. Three patients who tested positive for COVID-19 infection after returning to the US from South Africa were also assessed. Find Dosing And Administration Information For Moderna COVID-19 Vaccines.
The first drug approved to treat COVID-19 in late 2020 Remdesivir is the only antiviral treatment to receive full Food and Drug Administration approval - at least so far. Get Information to Help Determine if PAXLOVID Is Right for Your Patient. You may develop a. FDA-approved or -authorized vaccines boosters help protect you from COVID variants.
22 2021 the Food and Drug Administration FDA issued an Emergency Use Authorization EUA for a new medication to treat COVID-19. Paxlovid rebound happens when a person experiences worsening of COVID-19 symptoms after initially getting better after taking Paxlovid. Get the latest updates and find a healthcare provider prescribing the treatment. President Biden announced Thursday that he has contracted COVID and that he is taking Paxlovid to treat its symptoms.
Talk to Your Healthcare Provider About Starting PAXLOVID Treatment. By Kevin Loria. Paxlovid besteht aus zwei aktiven. This medication is called.
Developed by Pfizer a. Es gebe einen Anhaltspunkt für erheblichen Zusatznutzen gegenüber sonstiger Behandlung für. Paxlovid was 89 effective at reducing the risk of hospitalization or death according to data from the clinical trial that led to the drugs Emergency Use Authorization. Nach der Beschaffung von einer Million Packungen des Anti-Covid-19-Mittels Paxlovid.
You have been given a medicine for COVID-19. For people at high risk of severe COVID-19 including seniors and those with chronic health issues Pfizers Paxlovida prescription antiviral authorized for. Paxlovid is an oral antiviral treatment that can be taken at home to prevent high-risk COVID-19 patients from becoming sick enough to be hospitalized. Von dem Großeinkauf der Bundesregierung hatten bislang relativ wenige Patienten etwas.
Obwohl es möglich sein kann Paxlovid nach 2-25-tägigem vorübergehenden Absetzen der Antiarrhythmika zu beginnen ist dies vom praktischen Standpunkt aus. Werden an Corona erkrankte Patientinnen.
 |
| Ema Empfiehlt Covid 19 Medikament Paxlovid |
 |
| Nach Ema Zulassung Pfizer Aktie Verliert Dennoch Italien Beginnt Mit Auslieferung Von Corona Medikament Paxlovid Von |
 |
| Die Corona Arznei Die Leben Retten Kann Aber Kaum Verschrieben Wird |
 |
| Corona Infektion Schon Uber 17 000 Covid Medikamente In Wien Ausgegeben Wien |
 |
| Paxlovid Warum Lindners Wunderpille Kaum Jemand Nehmen Will Gesundheit Sz De |
Posting Komentar untuk "covid medikament paxlovid"